secure medical imaging cloud workflow solutions for clinical trial
-PHI under the HIPPA, ICH-GCP compliance, DICOM-CS, 21CFR part 11-
5-step cloud workflow solution in medical imaging
1. DICOM De-identification Re-identification (DICOM Masking and Trail)
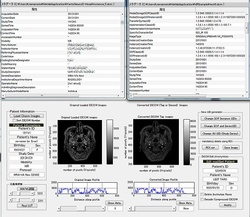
1. DICOM De-identification and Re-identification: Anonymized Database System
2. De-identifactation with Audit Trail (GCP Compliance)
3. DICOM Anonymization tool (Check difference between De-identification and Anonymization)
4. DICOM Rule for PHI and DICOM Supplement 142
5. Easy unique UID generation of Study UID, Series UID and SOP Instance UID 、
Screen Captured DICOM Patient is also cleaning using LISIT original developed image processing.
2. De-identifactation with Audit Trail (GCP Compliance)
3. DICOM Anonymization tool (Check difference between De-identification and Anonymization)
4. DICOM Rule for PHI and DICOM Supplement 142
5. Easy unique UID generation of Study UID, Series UID and SOP Instance UID 、
Screen Captured DICOM Patient is also cleaning using LISIT original developed image processing.
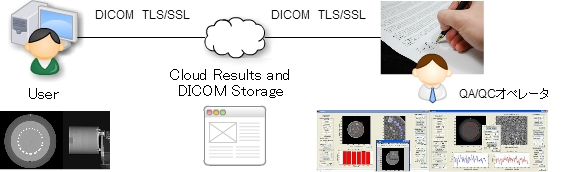
2. Cloud DICOM Image Quality Assurance and Quality Control Center
Cloud DICOM-QA / QC Service: Sponsor is only working to upload the image by imaging the phantom.
Requester can verify the authenticity of the report of the only imaging device to access the cloud.
Optimal protocol setting: keep radiation exposure as low as reasonably achievable (ALARA).
Requester can verify the authenticity of the report of the only imaging device to access the cloud.
Optimal protocol setting: keep radiation exposure as low as reasonably achievable (ALARA).
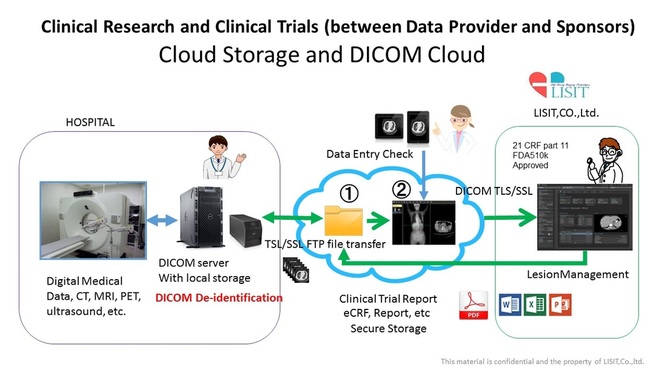
3. Data Transfer from Multicenter to Imaging CRO Phase I, II and III Clinical Trials
Lesion Managing under the 21 CRF part 11, Cloud storage of HIPPA compliant, FDA and CE Mark approval Software
LISIT manages clinical trial on demand / zero foot point system thorough medical secure cloud.
LISIT manages clinical trial on demand / zero foot point system thorough medical secure cloud.
- Our network system is high reliability not only FDA approved giving DICOM CS conforms but GCP complianced DICOM-IHE-CS.
- Significantly trial cost cut using Cloud System.
- Any VPN less, Server less and Software less to the client and facility of client. (Significant COST CUT for clinical trials)
- Cloud storage and disaster recovery (DR) of DICOM images and any data for clinical trials
- The range of user privileges will be granted to the flexible, you can view the images anywhere on any devices in tablet even smart phone.
- such as CT / PET / MRI image, you can select in the cloud database without having to access the imaging device.
- can be operated support and online conference on demand by remote support system.
- you can share or transfer the image to another user by granting user privileges.
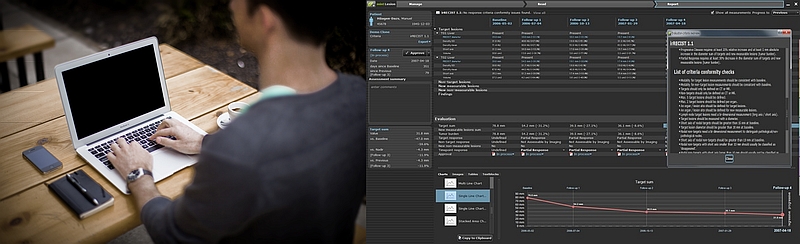
4. Lesion Management and Auto Criteria Service on Cloud
Features of mint Lesion (image evaluation system, which is designed to study a dedicated anti-cancer agent)
- Automatic evaluation of any criteria and covering the international standards and international guideline : RECIST 1.0, RECIST 1.1, WHO, Choi, irRC, Cheson, mRECIST HCC, EASL, RANO, PERCIST1.0, supports such as RECIST 1.1 modified irRC etc.
- licensing to pharmaceutical companies, CRO and Imaging CROs.
- You can assemble the manual evaluation criteria in the GUI using mint Lesion.
- Automatic DICOM cleaning function (it blocks .SC-DICOM, redundant DICOM images and the only image that has been established in the imaging scan protocol via automatic filtering)
- Imaging Core Lab (ICL) standards of FDA GMP, QSR, GCP, HIPAA, and Part 11 Electronic Records Standards compliance
- mint Lesion is designed IRC dedicated module that gives IRC reviewers, IRC imaging CRA, the authority in another role of IRC project manager
- Online central decision meeting correspondence using the trial cloud imaging trials dedicated cloud service
- Even in Phase III trials of more than Thousands of cases, mint Lesion produce the best performance with high-speed automatic judgment of any criteria of anti-cancer drug.
- Inter festival of clinical trial management and EDC system compatible
- Automatic generation function of eCRFs
5. Education System: Online ICH, GCP training, DICOM supplement 142 and medical imaging in clinical trials

- e-Pub online reading circle meetings of medical imaging in clinical trials.
- Computer system validation (CSV) of online check training
- DICOM communication training to be completed only in the cloud system
- Clinical trials dedicated DICOM masking (de-identification and re-identification)
- Remote maintenance of using Team Viewer and Soluto etc.
Adoption of International internship
Our Intern experience:
- Kitasato Medical Health Graduate School : 1 (Kanagawa Japan)
- Tokyo Institute of Technology, Information Science and Engineering: 1 (Tokyo Japan)
- Keio University Faculty of Pharmaceutical Sciences: 1 (Tokyo Japan)
- STRASBOURG UNIVERSITY, TELECOM PHYSIQUE, Strasbourg, France:1 (France )
- Waseda University Faculty of Science and Technology advanced one person: 1 (Tokyo Japan)